Electroplating is a process by which metal ions migrate via a solution from a positive electrode to a negative one. An electrical current passing through the solution causes objects at the cathode to be coated by the metal in the solution.
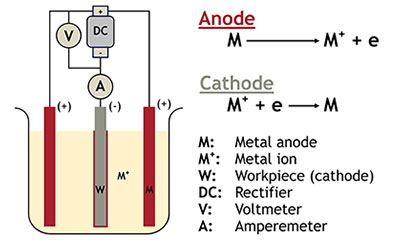
Common metals used in the electroplating process include black and silver nickel, chromium, brass, cadmium, copper, gold, palladium, platinum, ruthenium, silver, tin and zinc. We typically recommend using Grade S or N Nickel, cadmium pellets, CDA 101 OFHC Copper, brass alloys, tin anodes and zinc.
